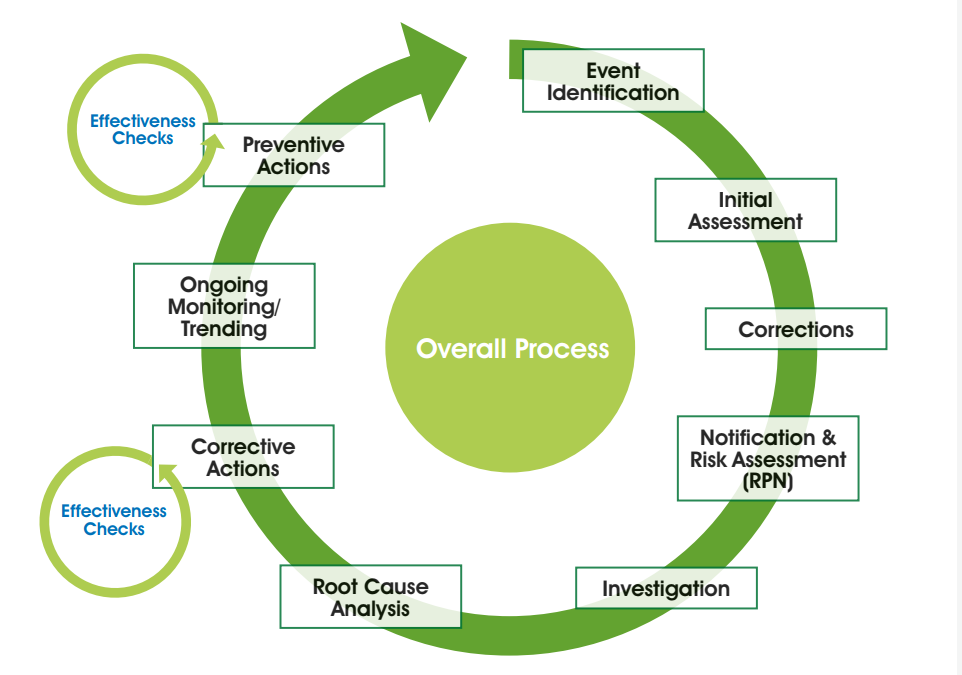
EFFECTIVE AND FAST APPROVAL OF INCIDENT INVESTIGATION REPORTS
Deviations, incidents or investigations – whatever you call them – require an efficient and effective system for review and approval.

Deviations, incidents or investigations – whatever you call them – require an efficient and effective system for review and approval.
With 56% of U.S. health care providers reporting to have changed patient care or delayed therapy as a result of drug shortages, John Johnson looks at the key reasons behind the issue and what you can do to stop it.

Imagine you are the Quality Director of a site manufacturing critical medicinal products or medical devices. You have a sickness absence rate of 50% of QA and QC staff. What do you do?
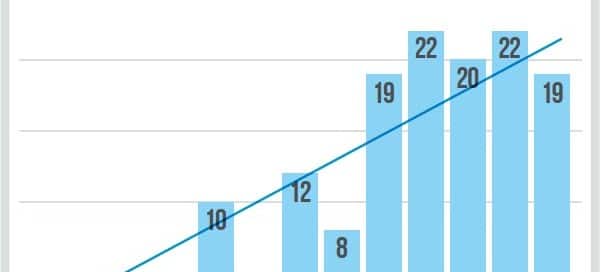

Why becoming more risk literate is so vital

A substantial number of cell and gene therapies (C>s) delivering a cure or at least durable health benefits are expected over the next 5 to 10 years – for example, MIT NEWDIGS researchers estimated that about 40 gene therapies may be approved by the end of 2022.